
Dose modifications can help optimize patient management to enable continued treatment when appropriate
Dose modifications for adverse reactions1*
If an adverse reaction (AR) listed below occurs, interrupt IMBRUVICA® therapy at each occurrence of the same AR.
Once the AR has improved to Grade 1 or baseline, follow the recommended dosage modifications below.1
Start at approved dose 420 mg
Once daily until disease progression or unacceptable toxicity
ADVERSE REACTION†‡
occurrence
1ST
2ND
3RD
GRADE 3 or 4: other non-hematological toxicities§
Restart at280 mg‖ daily
Restart at140 mg‖ daily
Discontinue
GRADE 3 or 4: neutropenia with infection or fever
Restart at280 mg‖ daily
Restart at140 mg‖ daily
Discontinue
GRADE 4: hematological toxicities
Restart at280 mg‖ daily
Restart at140 mg‖ daily
Discontinue
GRADE 2: cardiac failure
Restart at280 mg‖ daily
Restart at140 mg‖ daily
Discontinue
GRADE 3: cardiac arrhythmias
Restart at280 mg‖ daily
Discontinue
GRADE 3 or 4: cardiac failure
Discontinue
GRADE 4: cardiac arrhythmias
Discontinue
Certain types and severity of cardiac ARs require discontinuation after first occurrence for only IMBRUVICA®.
†See full Prescribing Information for Warnings and Precautions.
‡Grading based on National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) criteria, or International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria for hematological toxicities in CLL/SLL.
§For Grade 4 non-hematological toxicities, evaluate the benefit-risk before resuming treatment.
¶Evaluate the benefit-risk before resuming treatment.
For use with CYP3A inhibitors and inducers, and in patients with hepatic impairment, please see the full Prescribing Information. Consider the risks and benefits of anticoagulant or antiplatelet therapy when co-administered with IMBRUVICA®. Monitor for signs and symptoms of bleeding. Consider the benefit-risk of withholding IMBRUVICA® for at least 3 to 7 days pre- and post-surgery depending upon the type of surgery and risk of bleeding.
Dose modifications were designed to help manage ARs1
The outcomes of initial dose modification from pooled IMBRUVICA® patients (cardiac and non-cardiac related)1,2*
Study Design
- Pooled analysis from two single-arm clinical trials (Study 1118 and the iNNOVATE™ monotherapy arm) and one randomized, controlled trial (iNNOVATE™) included a total of 169 patients who were ≥18 years of age with WM treated with IMBRUVICA®
- Patients had either not been previously treated (iNNOVATE™) or had been previously treated (Study 1118 and iNNOVATE™ monotherapy)
Pooled IMBRUVICA®-treated patients:
N=169
29/169
Patients with a dose reduction for AEs per protocol
Dose modifications were per study protocol.
14/169
Patients with an AE leading to dose reduction per USPI*
In patients who both did and did not dose modify over the entire course of the long-term follow-up phase 3 trials
6%
10/169
of patients discontinued IMBRUVICA® due to AEs2
Of the 14 patients who required a dose reduction
Initial AE resolution:
For pooled IMBRUVICA®-treated patients
Initial AE that led to a dose modification was resolved in 100% of patients
AE recurrence:
Recurrence can take place after AE resolution.
No recurrence or recurred at lower grade
Recurred at same or higher grade
Study Context
- For the entire cohort, median overall follow-up for pooled analysis was 45 months.
- The median follow-up was 49.7 months for iNNOVATE™ (final analysis) and 14.8 months for Study 1118 (primary analysis).2
- Dose modifications were defined in the study protocol; patients may not have undergone IMBRUVICA® dose modification according to current recommendations. Dose modifications include a dose hold, followed by dose reduction.
- The timing of AE recurrence varied from patient to patient.
- Timing of AE outcomes, including resolution, recurrence, and discontinuation, was not part of the evaluation.
- Results from this analysis are descriptive in nature and have no implications regarding efficacy.
- These pooled analysis results are not included in the Prescribing Information for IMBRUVICA®.
From the USPI
- Five percent of patients receiving IMBRUVICA® across Studies 1118 and iNNOVATE™ discontinued treatment due to adverse reactions. Adverse reactions leading to dose reduction occurred in 14% of patients.1
AEs for which dose reductions are recommended in the USPI (grade 2 cardiac failure, grade 3 cardiac arrhythmia, grade 3-4 non-hematological AEs [excluding cardiac failure and cardiac arrhythmia], grade 3-4 neutropenia with infection or fever, and grade 4 hematological AEs).
See full Prescribing Information for complete dosage and administration details.
Dose modifications can help manage AEs so appropriate patients can continue to benefit1
iNNOVATE™ I+R (long-term analysis): Over 5 years of outcomes data with IMBRUVICA® post dose modification for AEs
iNNOVATE™ Arm A was a phase 3, randomized, double-blind, placebo-controlled study of ibrutinib or placebo in combination with rituximab and was conducted in treatment-naïve or previously treated patients with WM. The major efficacy outcome measure was PFS assessed by an IRC with additional efficacy measure of response rate.1
- Interpretation of this data is limited by the small sample size
- Dose modifications were defined by study protocol; presented outcomes are only those modified according to current recommendations. Dose modification includes both the dose hold and dose reduction of ibrutinib
Exploratory post hoc analyses: PFS in IMBRUVICA®-treated patients with or without dose modifications due to AEs2
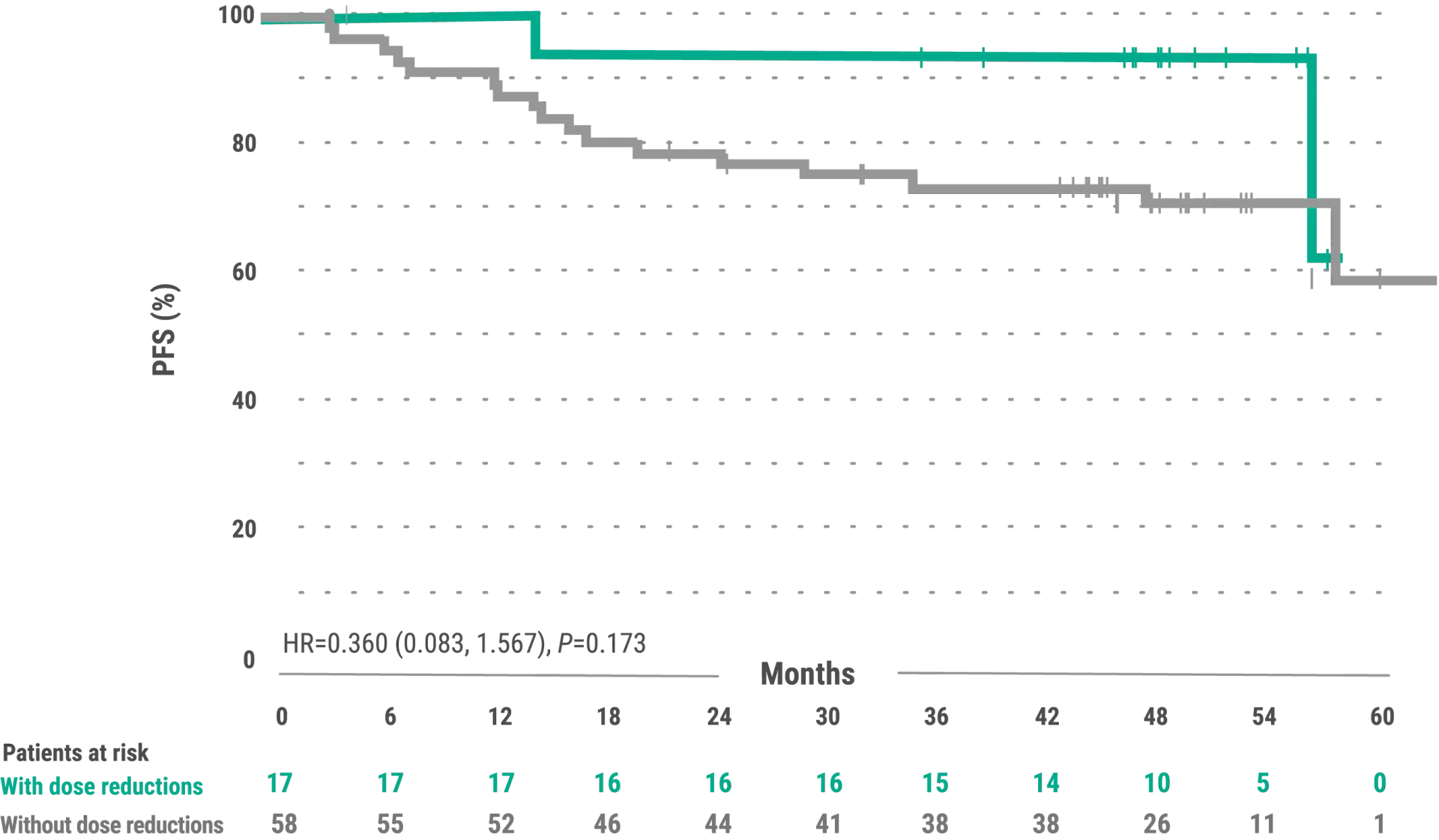
The exploratory analysis evaluated baseline demographics and clinical outcomes (PFS) in subgroups of patients with and without dose reductions due to AEs from the overall population of IMBRUVICA®-treated WM patients
- The median time to first dose reduction was 18.4 months (range, 4.0-40.6 months)3
- 17 patients dose reduced per USPI3
- Median follow-up was 49.7 (range, 48.7-51.6 months)2
- The median PFS for patients with dose reduction was not estimable (range, 14.7-57.4+ months; 95% CI: 56.3, NE). The median PFS for patients without dose reductions was not estimable (range, 3.4+-62.8+ months; 95% CI: 57.6, NE)3
- Median overall treatment duration was 48 months (range. 1-59 months); median treatment duration after dose reduction due to an AESI was 28.5 months (range, 1.1-51.4 months)3,4
- From the USPI: five percent of patients receiving ibrutinib across Studies 1118 and iNNOVATE™ discontinued treatment due to adverse reactions. Adverse reactions leading to dose reduction occurred in 14% of patients.1
- Results are based on a starting dosage of IMBRUVICA® 420 mg once daily. Kaplan-Meier curves (x time) have a limited sample size, potentially impacting PFS estimates2
- Subgroups of patients with and without dose modifications were not stratified for any baseline characteristics. Imbalances in baseline characteristics may exist between these groups
- Outcomes in the subgroup of patients with and without dose reductions in the overall population of all IMBRUVICA®-treated patients from iNNOVATE™ are exploratory post hoc analyses and were not powered for significance; comparative statistics are provided for descriptive purposes only
- Dose modifications for any reason were per protocol based and at the discretion of the physician
5-year iNNOVATE™ I+R long-term dosing results are not included in the Prescribing Information for IMBRUVICA®
Abbreviations
AESI=adverse event of special interest, AR=adverse reaction, CLL=chronic lymphocytic leukemia, CYP3A=cytochrome P450, family 3, subfamily A, IRC=Independent Review Committee, NCI-CTCAE=National Cancer Institute-Common Terminology Criteria for Adverse Events, NE=not estimable, PFS=progression-free survival, SLL=small lymphocytic lymphoma, WM=Waldenström's macroglobulinemia.
